Transcriptomic Profiling of Plaque Psoriasis and Cutaneous T-Cell Subsets during Treatment with Secukinumab - ScienceDirect

PDF) Repurposing of Secukinumab as Neuroprotective in Cuprizone-Induced Multiple Sclerosis Experimental Model via Inhibition of Oxidative, Inflammatory, and Neurodegenerative Signaling

Transcriptomic Profiling of Plaque Psoriasis and Cutaneous T-Cell Subsets during Treatment with Secukinumab - ScienceDirect

Transcriptomic Profiling of Plaque Psoriasis and Cutaneous T-Cell Subsets during Treatment with Secukinumab - ScienceDirect
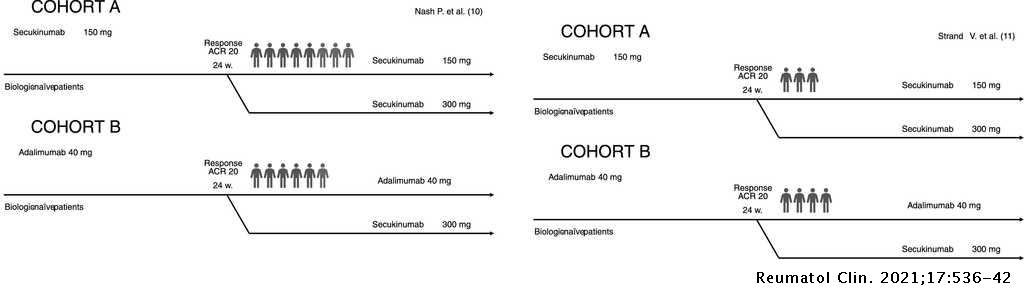
A cost-consequence analysis of the preferential use of secukinumab versus adalimumab for the treatment of psoriatic arthritis | Reumatología Clínica

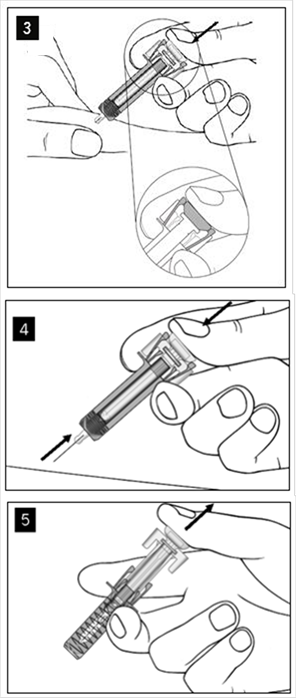
COSENTYX 150 MG INJEKČNÝ ROZTOK V NAPLNENEJ INJEKČNEJ STRIEKAČKE sol inj 1x1 ml/150 mg (striek.inj.skl.) - Príbalový leták
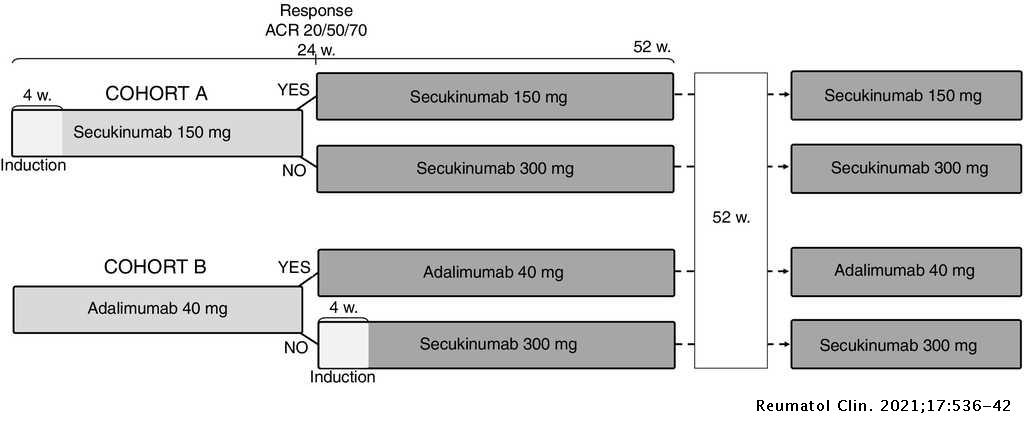
A cost-consequence analysis of the preferential use of secukinumab versus adalimumab for the treatment of psoriatic arthritis | Reumatología Clínica

Transcriptomic Profiling of Plaque Psoriasis and Cutaneous T-Cell Subsets during Treatment with Secukinumab - ScienceDirect
Instructions for Use Important Note Please read these instructions completely before using the GENOTROPIN MINIQUICK. If there

Transcriptomic Profiling of Plaque Psoriasis and Cutaneous T-Cell Subsets during Treatment with Secukinumab - ScienceDirect
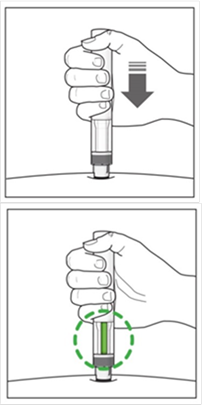
COSENTYX 150 MG INJEKČNÝ ROZTOK V NAPLNENEJ INJEKČNEJ STRIEKAČKE sol inj 1x1 ml/150 mg (striek.inj.skl.) - Príbalový leták