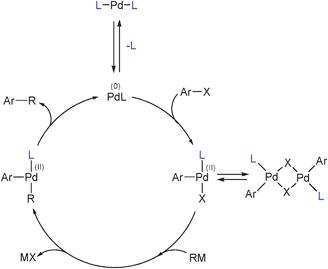
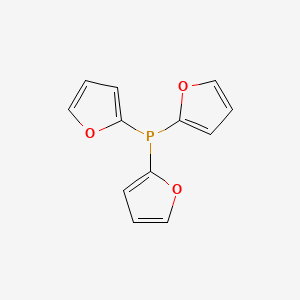
Palladium‐Catalyzed Heteroarylation and Concomitant ortho‐Alkylation of Aryl Iodides - Lei - 2015 - Angewandte Chemie International Edition - Wiley Online Library

Functional Group Transposition: A Palladium-Catalyzed Metathesis of Ar–X σ-Bonds and Acid Chloride Synthesis | Journal of the American Chemical Society

Palladium-Catalyzed Monoarylation of Arylhydrazines with Aryl Tosylates | The Journal of Organic Chemistry

Transition‐metal‐catalyzed Heteroannulation Reactions in Aqueous Medium - Dawood - 2022 - Asian Journal of Organic Chemistry - Wiley Online Library

Efficient and selective Palladium‐catalyzed Telomerization of 1,3‐Butadiene with Carbon Dioxide - Sharif - 2017 - ChemCatChem - Wiley Online Library

PDF) Palladium-catalysed reactions in solid phase organic synthesis | Johannes Köbberling - Academia.edu

Use of a bulky phosphine of weak σ-donicity with palladium as a versatile and highly-active catalytic system: allylation and arylation coupling reactions at 10−1–10−4 mol% catalyst loadings of ferrocenyl bis(difurylphosphine)/Pd - ScienceDirect

Cationic palladium( ii )-indenyl complexes bearing phosphines as ancillary ligands: synthesis, and study of indenyl amination and anticancer activity ... - Dalton Transactions (RSC Publishing) DOI:10.1039/D2DT01821G

Of the Ortho Effect in Palladium/Norbornene-Catalyzed Reactions: A Theoretical Investigation | Journal of the American Chemical Society

Functional Group Transposition: A Palladium-Catalyzed Metathesis of Ar–X σ-Bonds and Acid Chloride Synthesis | Journal of the American Chemical Society
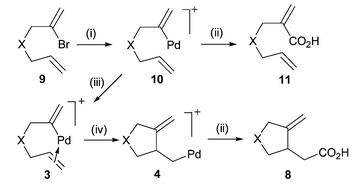
Palladium catalysed cyclisation– carbonylation of enynes to give cyclic γ,δ-unsaturated acids - Chemical Communications (RSC Publishing) DOI:10.1039/B300719G
Linear Triphosphines as Ligands for Metal Complexes Immobilization in Ionic Liquids: Palladium-Catalyzed Methoxylation of Alkyne
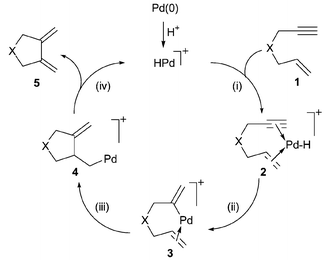
Palladium catalysed cyclisation– carbonylation of enynes to give cyclic γ,δ-unsaturated acids - Chemical Communications (RSC Publishing) DOI:10.1039/B300719G

Cationic palladium( ii )-indenyl complexes bearing phosphines as ancillary ligands: synthesis, and study of indenyl amination and anticancer activity ... - Dalton Transactions (RSC Publishing) DOI:10.1039/D2DT01821G

Applied Sciences | Free Full-Text | Arylation Reactions in the Synthesis of Biologically Important 2,5-Diaryl-1,3,4-Oxadiazoles

Selective Ortho Thiolation Enabled by Tuning the Ancillary Ligand in Palladium/Norbornene Catalysis | Organic Letters

Of the Ortho Effect in Palladium/Norbornene-Catalyzed Reactions: A Theoretical Investigation | Journal of the American Chemical Society
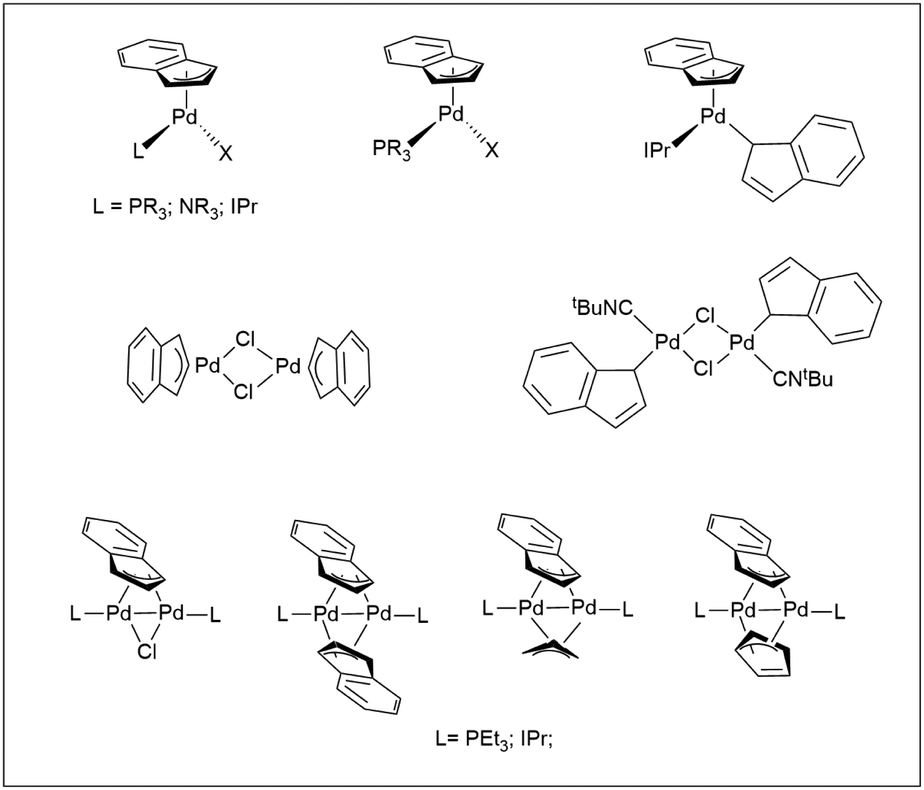
Cationic palladium( ii )-indenyl complexes bearing phosphines as ancillary ligands: synthesis, and study of indenyl amination and anticancer activity ... - Dalton Transactions (RSC Publishing) DOI:10.1039/D2DT01821G

Palladium-Catalyzed Heck-type Domino Cyclization and Carboxylation to Synthesize Carboxylic Acids by Utilizing Chloroform as the Carbon Monoxide Source
Synthesis of Diverse Heterocyclic Scaffolds by (3+3) and (3+4) Cycloannulations of Donor‐Acceptor Vinylcyclopropanes

Homogeneous and Heterogeneous Pd-Catalyzed Selective C–P Activation and Transfer Hydrogenation for “Group-Substitution” Synthesis of Trivalent Phosphines | Organic Letters

2-Phosphinoimidazole Ligands: N–H NHC or P–N Coordination Complexes in Palladium-Catalyzed Suzuki–Miyaura Reactions of Aryl Chlorides | Organometallics
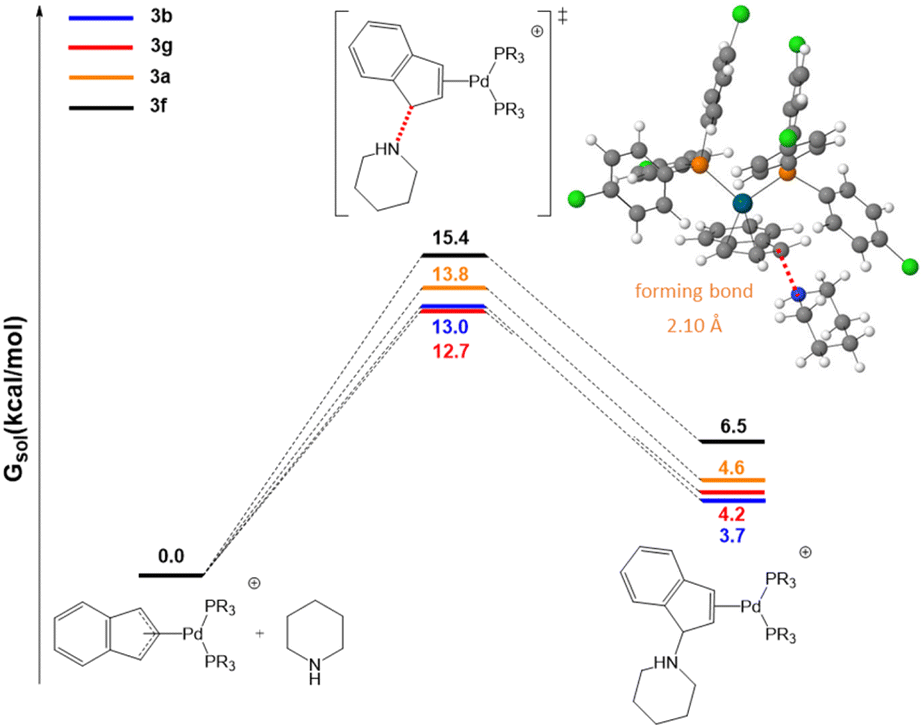
Cationic palladium( ii )-indenyl complexes bearing phosphines as ancillary ligands: synthesis, and study of indenyl amination and anticancer activity ... - Dalton Transactions (RSC Publishing) DOI:10.1039/D2DT01821G